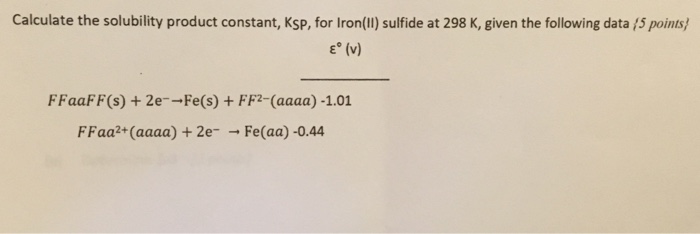
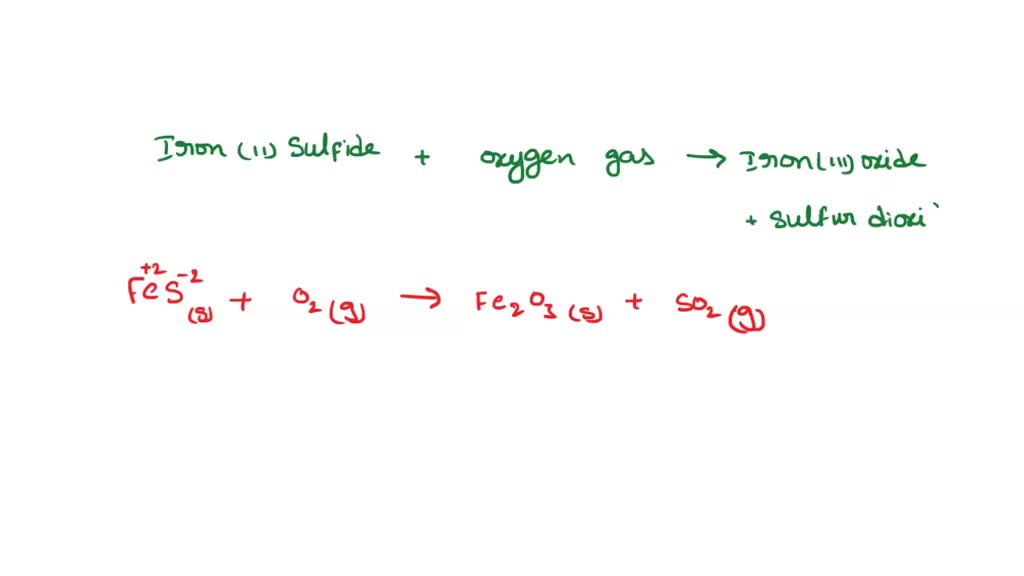Law of Conservation of Matter The Law of Conservation of Matter states that matter can neither be created nor destroyed.The Law of Conservation of Matter. - ppt download

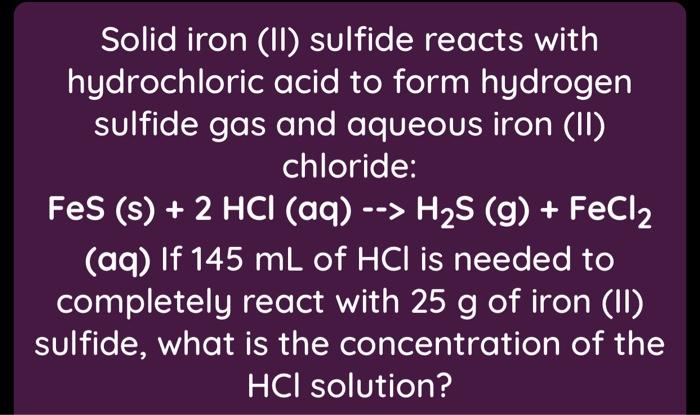
SOLVED:Iron(II) sulfide reacts with hydrochloric acid according to the reaction: FeS(s)+2 HCl(a q) ⟶FeCl2(s)+H2 S(g) A reaction mixture initially contains 0.223 mol Fes and 0.652 mol HCl. Once the reaction has occurred

Entry Task: Block 1 Nov. 13 th / 14 th NEW ENTRY TASK SHEET Provide the names or formulas for the following: Lead IV sulfide Zn 3 P 2 Mercury I nitride. - ppt download
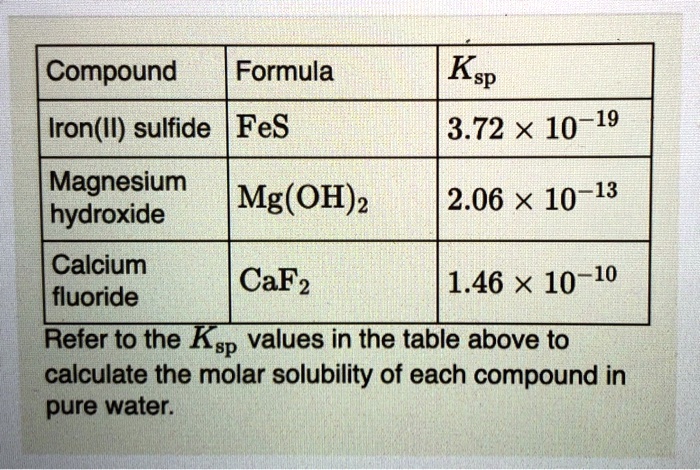
SOLVED: Compound Formula Ksp Iron(II) sulfide FeS 3.72 X 10-19 Magnesium Mg(OH)2 2.06 X 10-13 hydroxide Calcium CaF2 1.46 X 10-10 fluoride Refer to the Ksp values in the table above to